PrEP3 – Implementation study
Please note: The recruitment quota of this study has been full. Thanks for your support!
In view of the novel coronavirus outbreak (COVID-19), procedures for enrolment of participants for the study have been revised, so as to reduce the number of attendees for achieving social distancing. Participants are urged to pay special attention to the arrangement. We apologise for any inconvenience caused.
Aim and objectives
The project aims to develop a service model for PrEP delivery and test its operability in the real world setting. The objectives are, to:
- developing a PrEP service delivery model for sexual health needs of people at high risk of HIV infection;
- testing the practicability of integrating STI/HIV testing and HIV prevention and counselling in PrEP service;
- testing the incorporation of self-motivated arrangement for accessing PrEP
The Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee has approved this study (Ref. No.: 2019.345) and is authorised to access the participants’ records related to the study for ethics review purpose.
Study design

This is a 1-year study on PrEP usage. During the project period, Truvada® would be offered free to participants. You are required to attend the research clinic for a total of 5 or more visits over a one-year period. Before each appointment, you need to submit a test report confirming your HIV negative status within 1 month before the visit date. Diary, baseline, monthly and exit questionnaires have to completed during the study period. You are also advised to make arrangement for the following tests to be performed every 3-months: HIV antibody, testing for syphilis, gonorrhoea and chlamydia infections.
Eligibility criteria
To join the study, one must:
- be aged 18 or above
- be normally resident in Hong Kong
- be able to communicate in written and spoken English or Chinese
- have more than 1 episode of condomless sex (especially anal sex) in the preceding 6 months
- have been tested HIV antibody negative in the past 3 months
- have any of the following experiences in the past 6 months: chemsex/chemfun engagement, group sex, having multiple sex partners, STI diagnosis, having HIV+ve sex partner(s)
- be inclined to engage in high risk sexual activities in the coming months
- not be infected with hepatitis B
- have normal kidney function
- not be pregnant or planning to get pregnant (for female)
- not an injection drug user
Flow chart of the study
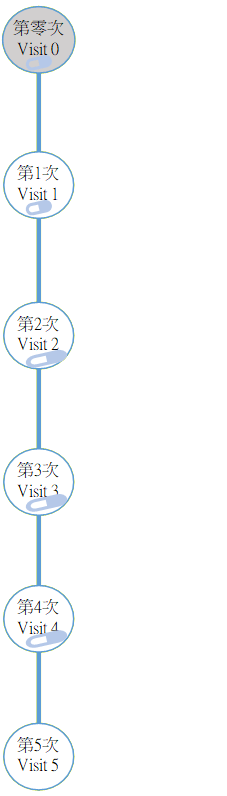
Before attending the baseline visit 0/1, you are reminded to self-arrange an HIV test apart from completing the baseline questionnaire online. At this clinic visit, you would see the doctor, have blood collected, and receive the prescription.
Subsequent Process :
- Take Truvada® every day (unless otherwise advised by doctor)
- Complete diary every week
- Complete an update questionnaire every month (takes around 5 mins)
- Test for HIV/STI and make appointment every 3 months
- Visit the clinic by appointment for consultation, blood sampling and obtaining Truvada®
- Some participants would be invited for an in-depth interview towards the latter part of the research.